
Nocita®(bupivacaine liposome injectable suspension)
Pain and dysphoria don’t have to be part of the post-op experience.* See the difference with Nocita, the only long-acting local anesthetic that controls post-op pain for up to 72 hours.
*In a field trial, Nocita reduced the need for post-op rescue pain treatment with opioids.
Why Choose Nocita?
- Up to 72-hour analgesia
- Controls pain to help post-op return to function
- Single-dose administration
- Less opioid use1
- Reduced opioid-associated side effects, including dysphoria

- Discharge patients sooner, possibly equalizing or reducing cost of care
- Potentially improving client satisfaction
- Helps patients recover comfortably even after they have gone home
Nocita Product Information

For relief of pain in dogs following CCL surgery and as a peripheral nerve block to provide regional postoperative analgesia following onychectomy in cats.

Injection

Bupivacaine liposome injectable suspension
How to Administer Nocita in Dogs
How to Administer Nocita in Cats
Administer 5.3 mg/kg per forelimb (0.4 mL/kg per forelimb, for a total dose of 10.6 mg/kg/cat) as a 4-point nerve block prior to onychectomy.
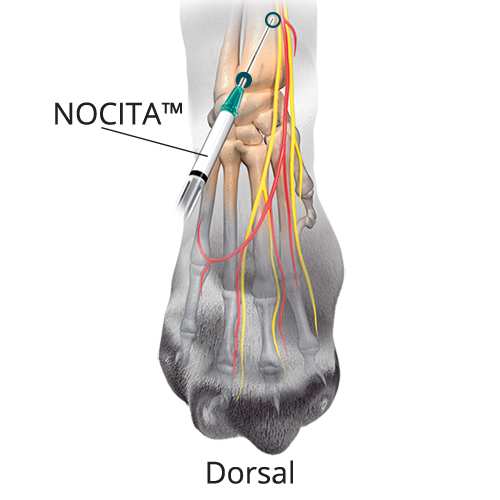 A: 0.14 mL/kg (35%) Superficial Branch of the Radial Nerve
A: 0.14 mL/kg (35%) Superficial Branch of the Radial NerveAt the center of the limb, on the dorsal aspect at the level of the antebrachio-carpal joint, insert the needle subcutaneously with the bevel up (•). Advance the needle subcutaneously and inject (o) adjacent to the confluence of the accessory cephalic and cephalic veins.
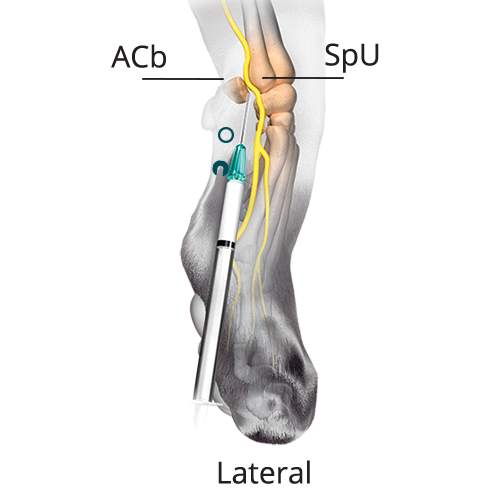 B: 0.08 mL/kg (20%) Dorsal Branch of the Ulnar Nerve
B: 0.08 mL/kg (20%) Dorsal Branch of the Ulnar NervePalpate a groove between the ACb in the base of the carpal pad and the SpU. Distal to this groove, insert the needle subcutaneously with the bevel up and advance the needle proximally. Inject once the tip reaches the midpoint of the groove.
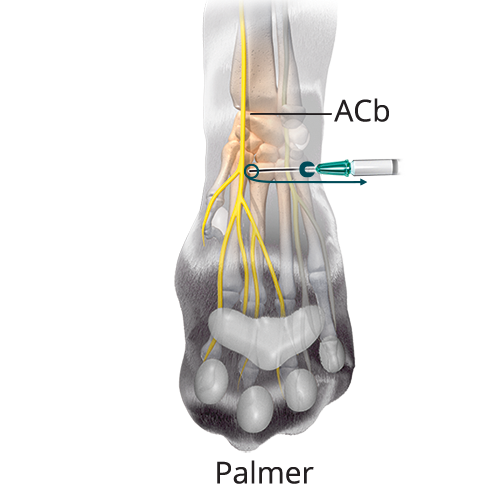 C: 0.16 mL/kg (40%) Median Nerve and Superficial Branch of the Palmar Branch of the Ulnar Nerve
C: 0.16 mL/kg (40%) Median Nerve and Superficial Branch of the Palmar Branch of the Ulnar NerveInsert the needle subcutaneously with the bevel up lateral to the distal tip of the accessory carpal pad and advance the needle medially two-thirds the width of the limb until the tip is located near the base of the first digit. Inject two-thirds of the volume at this point and the remaining volume while withdrawing the needle (solid teal arrow). Gently massage for five seconds.
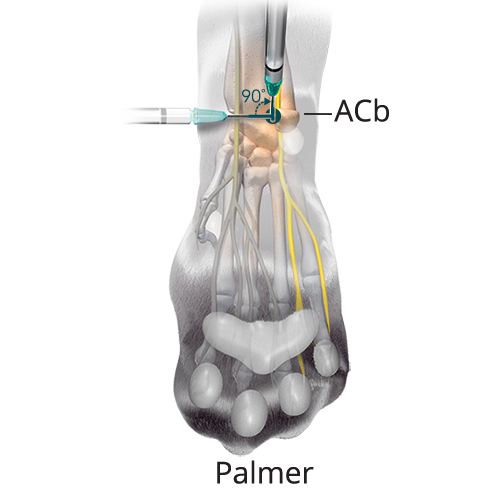 D:0.02 mL/kg (5%) Deep Branch of the Palmar Branch of the Ulnar Nerve
D:0.02 mL/kg (5%) Deep Branch of the Palmar Branch of the Ulnar NerveOrient the needle perpendicular to the long axis of the limb at the level of the ACb. Insert the needle subcutaneously and advance the needle laterally until it contacts the medial aspect of the ACb. Redirect the needle dorsally by rotating the needle 90 degrees. Advance it along the medial side of the ACb 2-3 mm until it penetrates the flexor retinaculum and inject.
Nocita®(bupivacaine liposome injectable suspension) Resources
Recovery care begins with Nocita
Incorporate Nocita into your postoperative pain protocols today.
Indication
For single-dose infiltration into the surgical site to provide local postoperative analgesia for cranial cruciate ligament surgery in dogs. For use as a peripheral nerve block to provide regional postoperative analgesia following onychectomy in cats.
Important Safety Information
NOCITA is for use in dogs and cats only. Do not administer concurrently with bupivacaine HCl, lidocaine or other amide local anesthetics. The safe use of NOCITA in dogs and cats with cardiac disease or with hepatic or renal impairment has not been evaluated. The safe use in dogs or cats younger than 5 months of age, that are pregnant, lactating, or intended for breeding has not been evaluated. The most common adverse reactions in dogs were discharge from incision, incisional inflammation and vomiting. The most common adverse reactions in cats were elevated body temperature and infection or chewing/licking at the surgical site. Click here to view full product label.





